Definition: "Treated article" is industry-speak for a solid or semi-solid object or product that has antimicrobial properties (that is not a medical device and does not claim to control microorganisms of public health concern).
Basically, "treated articles" are not regulated in the U.S. as are disinfectants (efficacy and safety data are not reviewed by the EPA). Such antimicrobial products are not allowed to make public health-related claims, including unqualified claims that they are "antimicrobial." For more information about how the EPA regulates treated articles, see:
http://www.epa.gov/pesticides/factsheets/treatart.htm
The confusion people experience when learning about and discussing antimicrobial consumer products is totally understandable! Part of the problem is that the term "antimicrobial" is very broad. Basically, anything that has a negative impact on microorganisms can be called "antimicrobial." Among antimicrobial consumer products, there is a range of activity, illustrated in the figure below.
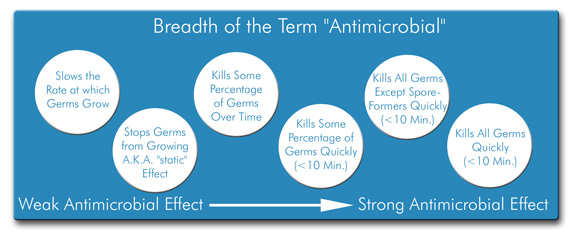
After considering this information, one might feel compelled to ask the question, "So products like antimicrobial pens, antimicrobial countertops, and antimicrobial surgical scrubs ...What kind of antimicrobial activity do they possess?"
It's an excellent question. The degree of antimicrobial activity in an "antimicrobial" consumer product varies by manufacturer and product but in general most treated articles demonstrate the kind of antimicrobial activity you'd find on the left half of the figure above. That is, companies have usually carried out tests on the antimicrobial products that demonstrate either growth-inhibiting or mildly "cidal" (germ-killing) properties.
Note: Disinfectants like Clorox bleach, Lysol Disinfectant Spray, etc. are EPA-registered antimicrobial pesticides and are not included in this discussion - visit http://www.antimicrobialtestlaboratories.com/information_about_disinfectants.htm
for information about disinfectants.
The most common test used for determining the antimicrobial activity of a treated article is the ASTM E 2149 Method. In short, the ASTM E 2149 test method isn't as realistic as it could be, and it is possible for a product to "pass" this test without producing an appreciable antimicrobial effect in "real-life" settings. Want to dig deeper? Visit www.antimicrobialtestlaboratories.com for more information about the ASTM E 2149 test method.
Many of the companies that make "treated articles" go the extra mile for their antimicrobial products. That is, whether they make the label claims or not, they develop products that have a public health impact and test them against relevant microorganisms using relevant test systems (many of which are designed on a custom basis).









